Whitepaper Explains Capabilities, Limitations of Botanical DNA Tests
DNA testing of botanical products is in its infancy, which has led to confusion and misuse of test results, according to a whitepaper that questions the validity of tests used by the New York Attorney General’s recent investigation into botanical supplements.
DNA testing of botanical products is in its infancy, which has led to confusion and misuse of test results, according to a whitepaper that questions the validity of tests used by the New York Attorney General’s recent investigation into botanical supplements.
The whitepaper, “The Capabilities and Limitations of DNA Barcoding of Botanical Dietary Supplements," was commissioned by supplement industry trade associations, and written by four scientists with experience testing natural products.
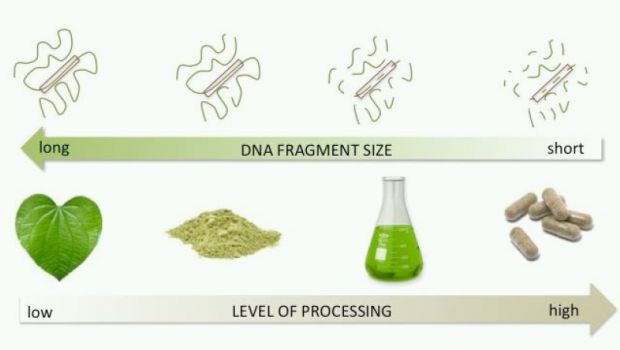
“Very little research has been conducted on the use of DNA barcoding for materials other than those that are fresh or living, especially on botanical extracts in dietary supplements where the DNA can be removed or degraded," the authors wrote. “Therefore, the use of DNA barcoding for finished dietary supplements is largely misunderstood and misapplied—even by those who claim to perform it. Despite several reports indicating the successful use of DNA barcode methods on botanical extracts, the results are erroneous and likely due to cross-contamination by raw or fresh materials either in manufacturing or in the laboratory conducting testing."
The whitepaper cites the recent investigation of botanicals conducted by the New York Attorney General Eric Schneiderman, and raises doubts on the conclusions reached by his office.
For instance, the authors wrote that DNA testing is not valid for botanical extract products because “Typically most, if not all, of the material containing cells (with the DNA) is removed during extraction, leaving the phytochemicals, but not the DNA," they wrote. The products tested in the Attorney General’s investigation are most likely made from extracts, industry has noted.
The whitepaper also pointed out:
Methods must be taken to ensure contamination of botanical samples does not occur,
Samples must be compared to a robust reference standard, and
Specialized training and experience in the field of plant species identification is required to obtain reliable results from botanical supplement testing.
However, the Attorney General has not released the data he used to question the products’ quality, despite industry’s call to do so. Thus, it is difficult to tell what measures the researchers took to ensure the tests were validated and samples weren’t adulterated.
“Numerous questions and concerns have been raised regarding the NY AG’s investigation, including the specific methods used and the experience of the scientists, and ultimately, the NY AG’s conclusions and actions based on the results," the authors wrote. “Without access to the complete methodology utilized, testing procedures and analytics employed, and the full test results, it is impossible to place any confidence in the announced outcomes of those tests. However, we can assert confidently that the conclusions—that a majority of supplements lacked any botanical at all—based only on the results of DNA barcoding tests were unjustified."
The white paper was commissioned by four trade associations representing the dietary supplement industry: the American Herbal Products Association (AHPA), the Consumer Healthcare Products Association (CHPA), the Council for Responsible Nutrition (CRN) and the United Natural Products Alliance (UNPA).
The authors of the whitepaper are Danica T. Harbaugh Reynaud, Ph.D., CEO, AuthenTechnologies LLC; Brent D. Mishler, Ph.D., director, University and Jepson Herbaria, University of California, Berkeley; James Neal-Kababick, director, Flora Research Labs LLC; and Paula N. Brown, Ph.D., director, natural health and food products research group, British Columbia Institute of Technology.
Be sure to attend the panel discussion “Adulteration & Your Role in Delivering Value to Consumers: Quality, Safety & Efficacy" at Ingredient Marketplace on April 8 from 9 to 10:30 a.m. in Orlando. Speakers Steve Mister, president and CEO, CRN; Mark Blumenthal, founder and executive director, American Botanical Council (ABC); and Dan Dwyer, managing partner, Kleinfeld, Kaplan and Becker LLP; will discuss brands’ role in ensuring a secure supply chain to delivers products to consumers that are free of adulterants, meeting their promise of quality, safety and efficacy.
Related content:
Video: The Best Way to ID Botanicals
Industry Offers Free Botanical Resources in Wake of NY AG Probe
About the Author(s)
You May Also Like






.png?width=800&auto=webp&quality=80&disable=upscale)