Such de facto pre-market approval of dietary supplements would harm ingredients such as CBD and NAC, while adding nightmarish procedural duties to an agency already missing in action, argues Kyle Turk of NPA.

This is part of Insider’s periodic updates from trade associations and other major stakeholders. These are meant to keep industry readers updated on these organizations’ activities and priorities, as well as to issue calls for action. These are op ed commentaries and represent the views from the authors and/or the organizations they represent, not the views of Insider or its editorial staff.
Whether FDA has what some misguided critics describe as “limited authority over supplements” and whether a mandatory product listing (MPL) is necessary has been the subject of much conjecture in recent years.
The Dietary Supplement Health and Education Act of 1994 (DSHEA) struck a critical balance between consumers’ access to safe and effective dietary supplements and the government’s interest in protecting the public from bad actors. The Food Safety Modernization Act (FSMA) was enacted later to amend the Federal Food, Drug and Cosmetic Act (FDCA) to make the agency more proactive. It provided FDA with new enforcement authorities designed to achieve higher compliance rates to respond better to problems when they occur.
During the December 2021 FDA commissioner nomination hearing for Robert Califf, M.D., Senate Health, Education, Labor and Pensions (HELP) Committee Chairwoman Patty Murray (D-Washington) stated:
“…when it comes to dietary supplements, people across the country who are looking to make healthy choices, are faced with a shelf full of products that make claims about their health benefits but lack rigorous oversight, because FDA does not have the authority to collect basic information about these products — or even to know what products are on the market. Families across the country buy and use these items every day. They deserve to know all the products… they entrust their health to are subject to type of careful FDA oversight they already trust to keep their food, drugs and medical devices safe.”
Sen. Murray’s comments are inaccurate concerning the regulatory oversight of the dietary supplement industry, and they should serve as a warning shot for the industry that she—along with Sen. Richard Durbin (D-Illinois) and Dr. Califf—are hellbent on reshaping our landscape in the name of “transparency.” It is also worth noting that there is some discussion of bringing up supplements as Congress considers user fee reauthorization, a proposal that we would vehemently reject.
Bad actors and effective use of current authorities
While responsible natural products retailers and manufacturers go to great lengths to ensure consumers have access to safe and well-regulated products, a few bad actors still try to sell products masquerading as dietary supplements. These companies do not represent the dietary supplement industry. And FDA knows many of them, including indicted dietary supplement companies and their executives, such as Blackstone Labs and Hi-Tech Pharmaceuticals. Unfortunately, you will still find products from such companies sold online and containing steroids like 1-DHEA and 1-androstenediol (click on the image below to see an example.) Are we really to believe MPL would deter such bad actors from selling steroids and other dangerous unapproved drugs to unsuspecting consumers?
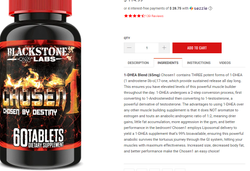
This isn’t a new concept; FDA has the enforcement mechanisms to act, but it picks and chooses when and where. In several cases, information regarding adulterated and counterfeit products has been presented to FDA, but the agency opted not to act. Just look at the agency’s inaction on import alerts. NPA presented FDA with opportunities to go after Chinese firms selling counterfeit beta-alanine, for which Natural Alternatives International has an agency-acknowledged new dietary ingredient (NDI) notification.
Would MPL solve these problems, or would it be more prudent to perform an in-depth analysis of FDA’s current authorities before handing them new ones? The agency has too often been missing in action (MIA) on implementation and enforcement of the law.
For instance, FDA inspected fewer domestic dietary supplement facilities in FY21 than it did in FY20 during the height of COVID-19. Would you say that’s an effective use of resources?
Leveraging NIH label database
Another concern is how FDA utilizes the National Institutes of Health’s (NIH) Dietary Supplement Label Database. This database was created at the direction of Congress in 2004 and has 129,943 labels that dietary supplement manufacturers and marketers voluntarily submitted. This is regularly updated to add new products and reflect formulations and other label changes for existing products. The product information included in the NIH database consists of the data FDA says it does not have, including:
Images of package labels
Name and form of ingredients
Amount of dietary ingredients
All label statements
On the market vs. off the market products
Consumers can learn more about the ingredients listed on the Supplement Facts label by clicking on the ingredient names (click on images below to see examples of listings.).
Over the years, the database has been redesigned with streamlined features and new delivery methods, including a mobile redesign and modernization. Most importantly, former Sens. Orrin Hatch (R-Utah) and Tom Harkin (D-Iowa) recommended the database be voluntary and under the NIH umbrella, not FDA’s, because they understood the ramifications of the agency becoming arbitrary and capricious.
But let’s take a step back and think about the consequences of MPL for the dietary supplement industry. We have experienced firsthand FDA’s illogical actions to preclude products from the market without justification, as well as the agency’s outright refusal to enforce its statutes and regulations.
CBD and NAC
Two products that come to mind are N-acetylcysteine (NAC) and cannabidiol (CBD). In both instances, FDA has explicitly stated CBD and NAC cannot be marketed in supplements because they were first approved (NAC) or studied (CBD) as drugs. Yet the NIH Dietary Supplement Database has nearly 350 CBD labels and over 800 NAC labels. This begs the question: What would be the fate of NAC or CBD if MPL was enacted?
MPL would subject the dietary supplement industry to a de facto premarket approval process, effectively eliminating the market for NAC, CBD and potentially other ingredients flagged by FDA. This goes against the principles of DSHEA and is the exact opposite of what so many stakeholders fought so hard to prevent.
MPL would be an administrative procedures nightmare for the industry.
Take CBD, for example. FDA believes the ingredient is precluded from being a dietary ingredient because it was first investigated as a drug. Thus, manufacturers or marketers for CBD products would not get their CBD supplements on the MPL. Consequently, retailers, e-commerce platforms and merchant services providers who sell or service these products despite not being on FDA’s MPL would fall out of compliance, subjecting themselves to significant financial penalties. It’s been estimated the CBD dietary supplement market has grown to more than $6 billion. If MPL were enacted, this market segment would be eliminated until FDA created a regulatory pathway for CBD. The industry has patiently waited for the last four years while more than thirty states have made their own regulatory pathways for CBD.
Then there is NAC, an ingredient that has been safely on the market for more than thirty years. If MPL had been enacted three or four years ago, NAC products would have suddenly been deemed ineligible for the product listing. Not only would those manufacturers, marketers, retailers, e-commerce platforms and merchant services providers be put in a bind, but NAC products would not reach consumers who have relied on these supplements for years.
Again, this would be an administrative procedures nightmare and go against DHSEA’s principles. DSHEA explicitly states the federal government should not take any actions to impose unreasonable regulatory barriers limiting or slowing the flow of safe products and accurate information to consumers. This is precisely what would happen if MPL were enacted. NAC, CBD and other ingredients under FDA administrative review would be removed from the market. Imagine trying to get your NAC or CBD product on the MPL during COVID-19.
FDA’s inaction and responsiveness have hit a new low. Even members of Congress, including Sen. Mike Lee (R-Utah) and Rep. Jeff Duncan (R-South Carolina), have been left in the dark on NAC, despite formally requesting more information on FDA’s determination. If Congress can’t receive a response from FDA, why would a company with an ingredient in question expect timely action? FDA has taken years to make determinations on CBD, and I suspect the same for NAC.
On average, a new medicine takes at least ten years to complete the journey from discovery to the marketplace. I recognize some stakeholders represent the drug industry in conjunction with supplements, so they might not fully understand the harm this would cause to the supplement industry. But does the dietary supplement industry want to open Pandora’s box and be regulated the same as drugs?
MPL warning to industry
If MPL becomes the law of the land, FDA will be the equivalent of your local Department of Motor Vehicles, where science and innovation take a back seat to endless administrative procedures. Without having to render an opinion on the legality and safety of a product, FDA will essentially have pre-market approval authority over dietary supplements, destroying the marketplace with non-final agency action.
Kyle Turk is the director of government affairs for the Natural Products Association (NPA), where he works with members to advance the organization’s federal and state public policy initiatives.
About the Author(s)
You May Also Like






.png?width=800&auto=webp&quality=80&disable=upscale)