Post-COVID, the number of dietary supplement facilities inspected by FDA has plummeted to just around 4%, according to Duffy MacKay of the Consumer Healthcare Products Association (CHPA). In this article, MacKay—a naturopathic doctor and veteran in the dietary supplement industry—outlines a proposal to fill gaps in FDA inspections.
.png?width=850&auto=webp&quality=95&format=jpg&disable=upscale)
This is part of Natural Products Insider’s periodic updates from trade associations and other major stakeholders. These are meant to keep industry readers updated on these organizations’ activities and priorities, as well as to issue calls for action. These are op ed commentaries and represent the views from the authors and/or the organizations they represent, not the views of Natural Products Insider or its editorial staff.
The scientific evidence supporting dietary supplements as part of preventive care continues to grow (BMJ. 2022;376, JAMA. 2012;308:1871-1880). However, doctors and medical societies have not fully embraced supplements because of concerns around inadequate regulatory oversight (J Clin Pharmacol. 2022;62:14-16). The responsible supplement industry has worked tirelessly to manufacture quality products and protect its reputation. Unfortunately, lack of FDA resources for enforcement makes it too easy for inexperienced companies or bad actors to operate without consequences.
An opportunity exists for supplements to play a greater role in health care, but to achieve this, the industry needs better assurances of supplement safety and quality. By authorizing third-party audits of dietary supplement manufacturers to FDA’s standards, the industry can raise assurances—and credibility—around product safety and quality of dietary supplements as they continue to be adopted as a mainstream part of self-care.
Dietary supplement safety and quality can be viewed in three key pillars. First, supplement manufacturers must establish the safety of ingredients and finished formulas through a variety of procedures. Second, manufacturing, packaging, labeling and holding of dietary supplements must be performed according to FDA regulations—called cGMPS (current good manufacturing practices)—to ensure a dietary supplement contains what is listed on the label and is not contaminated with harmful or undesirable substances. Finally, manufacturers must monitor and report post-market adverse events according to FDA regulations that apply to dietary supplements and over-the-counter (OTC) monograph drugs. When companies adhere to the three pillars of dietary supplement safety and quality, doctors and patients can feel comfortable they will achieve the scientifically established benefits of the ingredients.
The best way to assure manufacturers are adhering to the three pillars of supplement safety and quality is by requiring unannounced inspections of dietary supplement manufacturing facilities by qualified experts who can assess the facility for compliance with current federal regulations. Inspections include evaluation of the physical facility, staff qualifications, operating procedures, marketing claim review, ingredient review, laboratory procedures, safety documentation, complaint files and all other parts of manufacturing and supply chain management.
‘Audit tourism’
Over the past few years, FDA continues to report it lacks the necessary resources to achieve adequate inspection levels. FDA is responsible for inspecting over 8,000 foreign and domestic dietary supplement facilities, but the inspection gap is so significant that changes are needed. Pre-COVID, FDA inspected just 10% of the facilities per year, based on the Consumer Healthcare Products Association's (CHPA) conversations with the agency. Post-COVID, the number of facilities inspected has plummeted to just around 4%. Plain and simple, this inspection gap is unacceptable and does not provide broad assurances of quality and safety across the category to consumers or health care providers.
The gap in FDA supplement inspections has also led to an unsustainable environment for manufacturers. In an effort to protect their reputations from the absence of FDA inspections, some retailers are requiring additional third-party cGMP certifications. While this has its benefits, it has resulted in a patchwork of different third-party cGMP certification programs and a debate as to which one is best. Manufacturers face the additional resource burden of frequent inspections with different third-party certification standards, which has become so common in the supplement industry that it has been coined “audit tourism.”
Developing FDA-sanctioned program
Over the past several years, retailers, industry and public health experts have worked together through the Global Retailer and Manufacturer Alliance (GRMA) to harmonize third-party cGMP requirements into a single, publicly accredited standard for dietary supplements called NSF/ANSI 455.2. While these advances in self-regulation are great for the industry, it’s time to go even further by developing an FDA-sanctioned program that authorizes third parties to conduct audits to FDA’s standards that can take the place of an inspection conducted by FDA. FDA can review third-party-generated audit reports and use the information to prioritize its limited inspection resources to target high-risk facilities.
We believe a qualified inspector should visit all dietary supplement facilities to evaluate infrastructure, staff, ingredients and processes against legally required activities that are designed to ensure the identity, purity, quality, strength and composition of dietary supplements. Stakeholders across the industry would benefit from these third-party inspections. Consumers benefit from improved product safety and quality across the category. FDA benefits from audit reports from all supplement manufacturing facilities. And industry benefits from a level playing field where all manufacturers are required to invest adequate resources to adhere to manufacturing and testing standards.
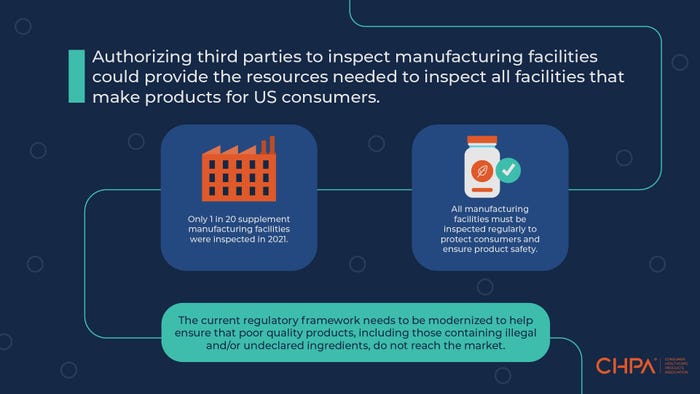
This proposal is not out-of-the-box thinking for FDA. The agency already has several programs that authorize third-party “certification bodies” that conduct food safety audits and issue certifications. For example, the Voluntary Qualified Importer Program (VQIP) is an established model used to help ensure imported foods are produced in accordance with U.S. food safety standards. This same model can be applied to dietary supplements to ensure they are produced in accordance with cGMP standards.
Broader regulatory reform
Third-party authorization of cGMP audits is only one priority within a broader plan to advance dietary supplement regulation through modernization of the Dietary Supplement Health and Education Act of 1994 (DSHEA). In addition to annual cGMP audits of all facilities, companies should be required to provide FDA with a list of product labels to generate a database of all dietary supplement products and ingredients on the market. A mandatory product listing could provide FDA a second tool that can help identify and remove illegitimate and illegal products from the market.
With expanding evidence that supplements can help people stay healthy and reduce health care costs, we anticipate dietary supplements will continue to play a larger role in health care. Supplements have a major opportunity to be included as managed-care benefits, tax-deferred benefits and to be recommended by providers, but the industry must provide stronger assurances of product safety and quality. Modernizing regulatory oversight to allow for inspections of all facilities—on top of broader regulatory reforms—would be a major step in the right direction for our growing industry.
Naturopathic doctor Duffy MacKay is the senior vice president of dietary supplements at the Consumer Healthcare Products Association (CHPA), where he leads the association’s dietary supplement scientific, policy and legislative initiatives. His career in supplements spans over 25 years and includes serving as a senior executive and scientist at leading dietary supplement companies, including CV Sciences, Nordic Naturals and Thorne Research.
About the Author(s)
You May Also Like






.png?width=800&auto=webp&quality=80&disable=upscale)