Specnova's proprietary liposomal validation programs ensure superior ingredient protection and stability, maximum bioavailability, and state-of-the-art verification technology.
March 31, 2023
Sponsored by Specnova
A nutraceutical product is only as good as it is bioavailable–its ability to deliver the nutrient payload for the intended benefit. That’s why Specnova offers two distinct programs to ensure top-quality stability and bioavailability: a proprietary manufacturing technology called LipoVantage™ combined with protective AG2™ Shield Technology and a comprehensive quality control system called TruLiposome™ Validation.
The natural supplement industry, though increasingly popular with consumers of all ages and lifestyles, still struggles with a significant hurdle: challenges with bioavailability and optimization. While not all ingredients have poor availability, some of the most popular and beneficial natural ingredients do, including Vitamin C, CoQ10, and curcumin to name a few. Indeed, the body is often unable to absorb active ingredients in quantities that produce results. Typically, brands solve for this by over-formulating, using more of the active ingredient to compensate for low bioavailability (which can get very expensive and lead to GI distress for some consumers and no matter how much you add, you still have low bioavailability and it won’t get into your body.
For this reason, many formulators are turning to liposomal delivery systems. Liposomes are tiny, globular, bi-layer structures with an aqueous core, which can be filled with water- and fat-soluble ingredients. This method of delivery offers some huge advantages including high bioavailability, protection against the harsh environment of the GI system which allows more of the active component to be absorbed, and cost-effectiveness – since more active ingredient is absorbed formulators need less of the ingredient to be effective. However, formulators must also consider the delicate nature of liposomes, which can rupture during manufacturing, causing the active ingredient to leak out reducing the benefits of the liposome. To solve for this, Specnova offers LipoVantage, a delivery system offering double liposomal encapsulation, which protects both the structure of the liposome and the active ingredient inside.
What is LipoVantage™ ?
LipoVantageTM technology is a state-of-the-art liposome creation process that offers superior bioavailability and potency. This proprietary manufacturing technique uses AG2™ Shield Technology to encapsulate the entire liposome, helping to maintain its structure and decrease the possibility of leakage.
AG2 Shield Technology, which consists of gum arabic, a natural and non-GMO plant polysaccharide from the acacia tree, and alginate from brown algae, offers a variety of benefits. First, and most obviously, it protects the liposome and increases stability in the body, therefore improving the bioavailability of the active components. Plus, LipoVantage liposomes created with AG2 are considered nano-molecules, and their extremely small size further contributes to superior solubility and permeability. AG2 also increases manufacturing performance by yielding a stable powder for easy blending, encapsulation, and formulation, with minimal taste and odor, and improved water solubility—even for fat-soluble ingredients.
These benefits offer significant improvement for the oral delivery of poorly soluble nutraceutical ingredients, with impacts stretching across the functional food and dietary supplement industry. But Specnova takes this innovation one step further by conducting extensive quality control testing to ensure that its liposomal ingredients are truly liposomes—this is the only way to guarantee maximum effectiveness.
TruLiposome™ Validation
Specnova is setting new standards for liposomes in the natural products industry with its exclusive TruLiposome™ 4-Step Validation process. True liposomes must meet Specnova’s four criteria in order to provide optimum effectiveness. Specnova is the only company to validate liposomes with all four methods.
To achieve validation, liposomes must meet the following requirements:
Size
Specnova scientists test the size of the liposome, to ensure it is between 100 and 200 nm—this is the size required in order to be absorbed by the small intestines effectively. Utilizing dynamic light scattering analysis (DLS), Specnova confirms this small particle size for two significant benefits. First, as the size of the particle decreases, bioavailability generally increases; second, smaller particles offer a more stable suspension due to increased surface-to-volume ratio.
Encapsulation Efficiency
In order to meet the specifications of TruLiposome Validation, the liposomes in the sample must display strong encapsulation efficiency to keep the active ingredient inside the liposome.
Shape
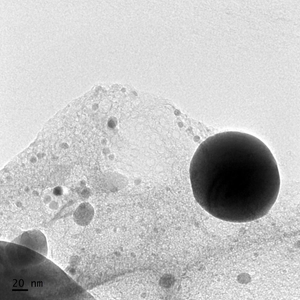
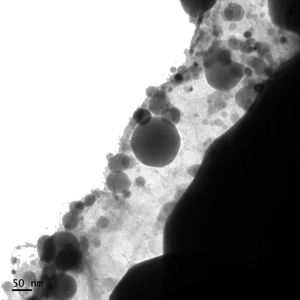
Liposomes must be spherical in shape to achieve TruLiposome Validation. Cryo TEM images and a scanning electron microscope (SEM) are employed to confirm this shape and structure.
Dispersion and Leak Protection
Liposomes verified via TruLiposome Validation must be well dispersed and free of leakage. A Cryo Transmission Electron Microscope (TEM) is used to show that the liposomes are well dispersed, and there is minimal liposomal debris which might indicate vesical rupture.
Leakage is assessed via zeta potential measurements, which essentially indicate the strength of the encapsulation of the active ingredient within the liposome. The liposome’s pH and ionic strength are also assessed, as protection may be needed during the low pH environment of the stomach and high pH in the presence of bile salt. Confirming the strength of LipoVantage and AG2 technology via zeta testing means there is less potential for leakage and more potential for higher stability in the body, during manufacturing, and on the shelf. The result is a slow-release active component that is more bioavailable.
The formulation advantage
LipoVantage technology utilizes ingredients that are vegan, non-GMO, and -free -of all major allergens including cereals, eggs, fish, soybeans, milk, nuts, mollusks, and more, and is appropriate for a range of product applications including gummies, chews, functional foods, capsules, tablets, powders, and ready-to-drink beverages. Formulators can rest easy knowing that the liposomes developed using LipoVantage Technology are confirmed with the extensive TruLiposome Verification process utilizing DLS, SEM, TEM, and zeta potential testing. This is something that no other company does, and is offered to Specnova customers for guaranteed efficacy and bioavailability.
References:
Pinar Erkekoglu, A. “General Overview of Glutathione, Glutathione Transport, and Glutathione Applications.” DOI: 10.5772/intechopen.81594.
Meister A. “Biosynthesis and function of glutathione, an essential biofactor.” J. Nutrit. Sci. Vitaminol. 1992; Spec No: 1-6.
Akbari-Alavijeh, S et al. (2020). “Encapsulation of food bioactives and nutraceuticals by various chitosan-based nanocarriers. Food Hydrocolloids;105:105774. https://doi.org/10.1016/j.foodhyd.2020.105774
Akbarzadeh, A et al. “Liposome: Classification, preparation, and applications.” Nanoscale Research Letters. 2013; 8(1), 1-9. https://doi.org/10.1186/1556- 276X-8-102
Allen, T. M. et al. (2013). “Liposomal drug delivery systems: From concept to clinical applications.” Advanced Drug Delivery Reviews. 2013;65(1):36-48.
Read more about:
Co branded articlesYou May Also Like




.png?width=800&auto=webp&quality=80&disable=upscale)