Establishing clear expectations about documentation and procedures plays a key role for contract manufacturers and own-label distributors in verifying FDA compliance with current good manufacturing practices (cGMPs) of dietary supplement products.
June 16, 2016
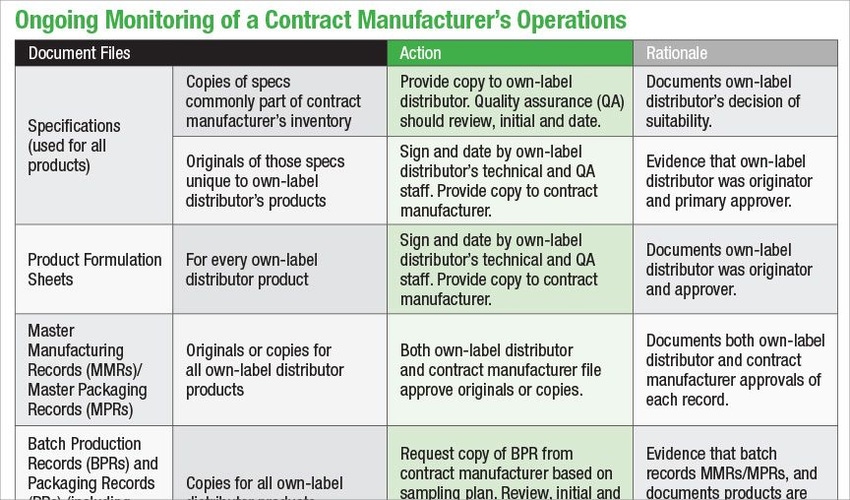
The increasing reliance on contracted services by both large and small marketing dietary supplement companies has created challenges in understanding and meeting both individual and mutual responsibilities. Working together to understand and meet those responsibilities goes a long way to achieving success for each party in terms of reliability of service, uninterrupted supply of high-quality product, a sustainable and profitable business relationship and avoidance of potential FDA issues.
For a host of resources on potential contract manufacturing partners, click the text link to visit the SupplySide & Vitafoods Global Storefronts.
About the Author(s)
You May Also Like




.png?width=800&auto=webp&quality=80&disable=upscale)