NBTY’s Rexall intends to set aside US$7.5 million to pay cash awards to class members, attorneys’ fees, and incentive awards of $5,000 each to class representatives.
February 16, 2016
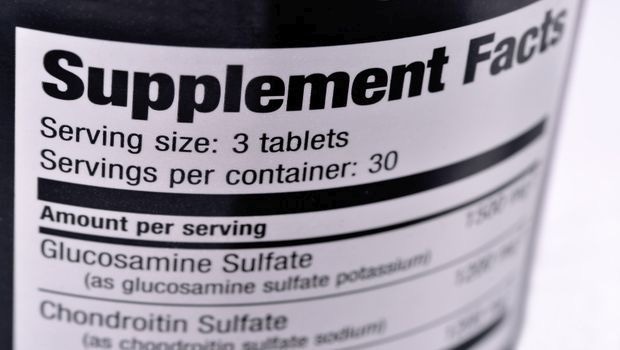
NBTY Inc. may be close to resolving putative class-action lawsuits challenging statements that tout the benefits of glucosamine and chondroitin joint health supplements.
A federal judge on Feb. 1 preliminarily approved a settlement, which would resolve lawsuits pending against NBTY, its subsidiary Rexall Sundown, Inc., and a number of retailers including Costco Wholesale Inc., CVS Pharmacy Inc. and Target Corp.
Rexall intends to set aside US$7.5 million to pay cash awards to class members, attorneys’ fees, and incentive awards of $5,000 each to class representatives, according to court records. The company also will pay $1.5 million for the costs of notice and administration.
The proposed settlement covers a class of roughly 15 million households, and plaintiffs anticipate reaching 74 percent of the class through its notice.
Members of the class are eligible for an award of $8 per bottle of the covered products and up to $104 per household reflecting the purchase of 13 bottles. Perhaps most significantly, the defendants have agreed to refrain from making statements that the dietary supplements help build or repair cartilage.
This is the second time NBTY has reached an agreement to settle the lawsuits. The U.S. Court of Appeals for the Seventh Circuit reversed the prior agreement following an appeal by class members.
The new agreement “appears to be a fair, reasonable, and adequate settlement of the litigation, and is in the best interests of the class in light of the factual, legal, practical, and procedural considerations raised by the litigation," James B. Zagel, a federal judge in the Northern District of Illinois, wrote in his order.
The judge established June 30 as the date for a hearing on the fairness of the settlement and Sept. 28 as the deadline for filing claims.
Plaintiffs’ lawyers and NBTY, which disclosed the court’s decision in a recent Securities and Exchange Commission filing, had no immediate comment.
Rexall manufactures glucosamine joint health supplements that are sold under brand names such as Osteo Bi-Flex. The company also makes for Target and other retailers private-label brands of glucosamine and chondroitin joint health supplements.
The putative class action lawsuits date back to 2011 and were filed in Illinois and on both coasts: California and Massachusetts. NBTY has faced allegations that representations regarding certain dietary supplements—“including most significantly the claim that glucosamine and chondroitin will rebuild or renew cartilage"—are false and misleading, according to plaintiffs’ May 14, 2015 memorandum seeking preliminary approval of the current settlement. The claims, which were brought under various state consumer protection laws, don’t relate to safety or physical injury.
Although NBTY has denied the allegations, the settlement requires the removal of certain statements affecting dozens of products and various brands such as Nature’s Bounty, Duane Read, Flex-a-Min and Life Fitness, the court documents noted.
Rexall must refrain from making the following statements on labels of products covered under the agreement: “fixing, mending, reconditioning, rehabilitating, increasing, developing, building, repairing, rebuilding, renewing, regrowing, adding, regenerating, or rejuvenating cartilage," according to plaintiffs’ memorandum.
The prohibition excludes “other claims regarding the effect of the covered products on cartilage, or … other types of structure/function claims, such as claims that the covered products support, protect, or promote joint comfort, mobility, or health."
Commenting on the anticipated “battle of experts" if the cases advanced to trial, the plaintiffs’ lawyers declared in the memorandum, “The efficacy of the products has been the subject of scientific and medical debate over the last decade, and while Plaintiffs believe that the weight of the scientific evidence is in their favor, as with any other trial there is the risk that a jury would side with Defendants."
Under the previous agreement as approved, Rexall was required to pay $5.63 million, including $1.93 million to class counsel, $179,676 in attorney expenses, $1.5 million in notice and administration costs, $1.13 million to the Orthopedic Research and Education Foundation, $865,284 to the 30,245 class members who submitted claims, and $30,000 to the six named plaintiffs.
But the Seventh Circuit characterized the agreement as a “selfish deal between class counsel and the defendant" that “disserves the class."
The appeals court objected to the notice and claim forms, and pointed out the award to claimants ($3 for up to four bottles, or $5 for up to 10 bottles with proof of purchase) was modest.
“Class counsel shed crocodile tears over Rexall’s misrepresentations, describing them as ‘demonstrably false,’ ‘consumer fraud,’ ‘false representations,’ and so on, and pointed out that most of the consumers of Rexall’s glucosamine pills are elderly, bought the product in containers the labels of which recite the misrepresentations—and number some 12 million," Circuit Judge Richard Posner wrote in the Nov. 19, 2014 opinion reversing the judgement on behalf of a three-judge panel. “Yet only one-fourth of one percent of these fraud victims will receive even modest compensation, and for a limited period the labels will be changed, in trivial respects unlikely to influence or inform consumers. And for conferring these meager benefits class counsel should receive almost $2 million?"
The parties have sought to address the Seventh Circuit’s concerns in the new agreement. For instance, claimants can recover up to $104 per household without proof of purchase, plaintiffs’ counsel noted. Also, the claims process has been simplified, Rexall’s total liability doesn’t hinge on the number of claims filed, and the new agreement includes a permanent prohibition on certain statements, according to plaintiffs’ memorandum.
“The only way that Rexall may again be allowed to make these claims is if it becomes aware of scientific support for any of the prohibited representations and obtains permission from the court, after notice to class counsel, that it may again make the prohibited representations," plaintiffs’ lawyers noted in a filing.
To read older court documents related to the case including the previous class notice, go here.
You May Also Like




.png?width=800&auto=webp&quality=80&disable=upscale)