Lipids
thumbnail
Healthy Living
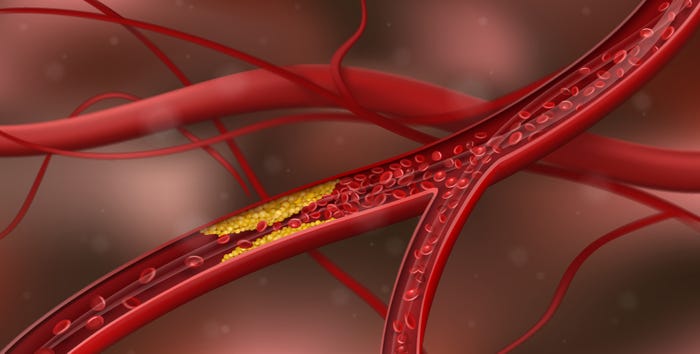
Study reinforces that high omega-3 levels can fight frailty in elderlyStudy reinforces that high omega-3 levels can help seniors avoid getting frail
A new study using data from a large population cohort has found that high omega-3 levels correlate to less frailty among elderly subjects.
Subscribe and receive the latest insights on the health and nutrition industry.
Join 37,000+ members. Yes, it's completely free.




























.png?width=300&auto=webp&quality=80&disable=upscale)






















.png?width=800&auto=webp&quality=80&disable=upscale)